Ankur Saxena, VP of Product Development of EUPHOR, the only software solution specifically designed for the chemical industry to manage complex compliance programs, discusses the importance of implementing robust IT systems to turn regulatory challenges into opportunities for streamlined compliance processes.
At EUPHOR, we are strong believers that appropriate IT solutions make the difference when dealing with strategic business processes. This is why we find it surprising that many chemical organizations lack the digital tools to efficiently manage and track their global compliance program.
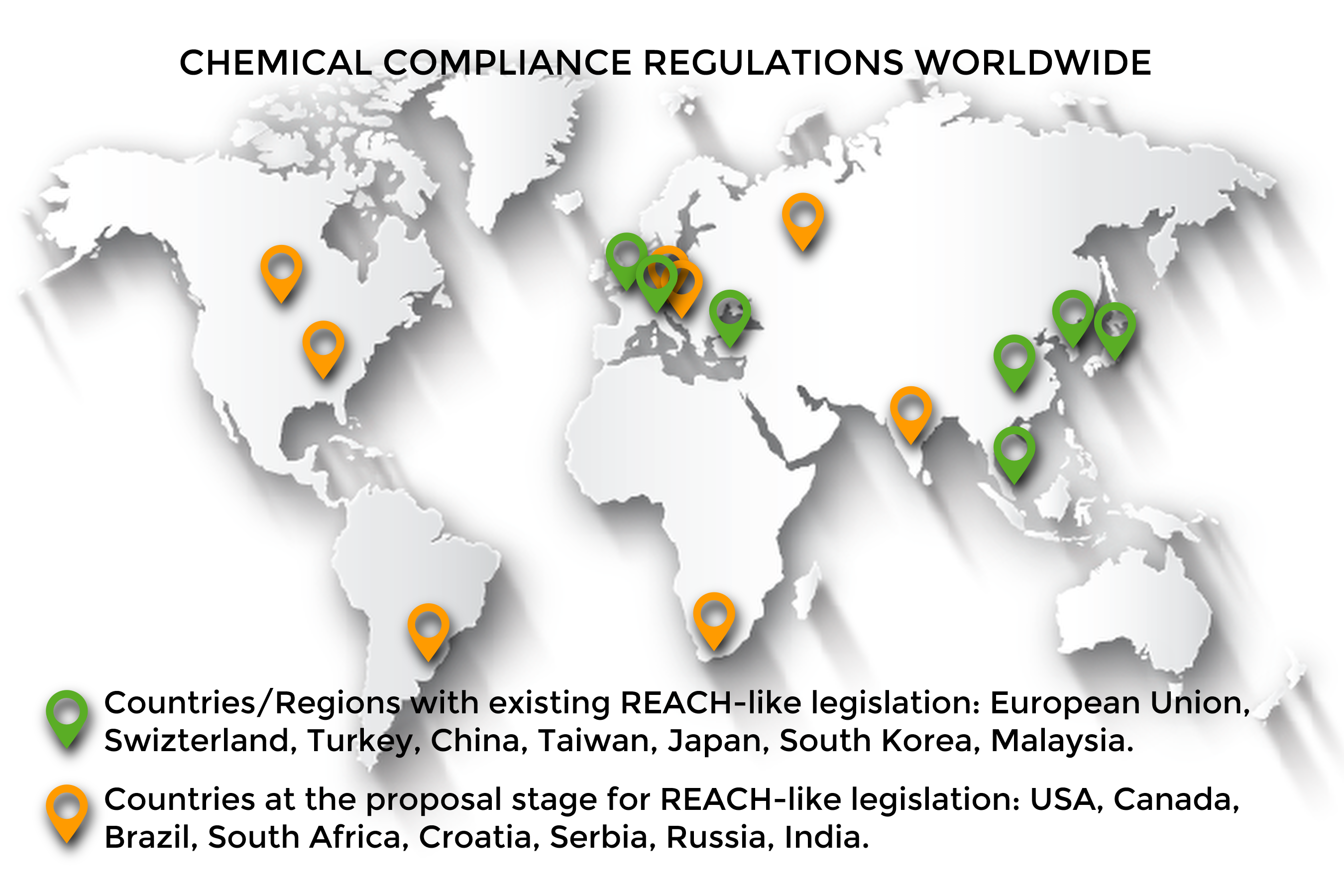
Most compliance departments still use multiple applications such as Excel spreadsheets, worksheets, different document sharing platforms and email-based tools leading to disparate processes throughout the supply chain and resulting in high margins of errors. As Global Compliance becomes more complex and widespread, we wonder why Compliance isn’t yet digitalized. Shouldn’t companies level the field between Product Stewardship and other strategic departments such as Sales, R&D or Finance which often benefit from state-of-the-art systems? Regulatory Compliance Environment: Ever-changing global dynamics. Whether we refer to chemicals in our mattress or shampoo, some questions require answers: how clean and safe is it? are there safer alternatives? This is what governments are trying to find out by implementing Compliance Regulations.
Controls imposed on the chemical industry are countless. Most countries have now implemented legal frameworks affecting chemical-based products (EU-REACH, K-REACH, China REACH, etc.). Although these regulations share the same goal, protecting users and the environment, they are ever-changing living documents that differ with regards to their specific roadmaps and requirements. This is where lies the biggest challenge for globally-active organizations: it creates an unstable business environment where they must track numerous laws while managing all the activities involved within each program. These regulations also share a common consequence for organizations failing to register: “NO DATA, NO MARKET”. Failure to meet requirements may result in companies facing non-compliance issues and ultimately having to withdraw from regulated markets. It is therefore vital to consider Compliance as a strategic aspect of globally-active organizations.
How EUPHOR Turns Compliance Challenges into Solutions
IT is increasingly called upon to provide organizations a set of tools to gain a greater situational awareness and control over Global Compliance. With the objective to digitalize compliance, EUPHOR was designed to manage an organization’s entire compliance program using a single platform. EUPHOR harmonizes disparate and complex processes by providing 4 key traits necessary for successful compliance:
- Automation: By automatically generating workflow based on governmental guidelines and the organization’s own data, EUPHOR permits to plan ahead and have a global picture of compliance processes from start to finish.
- Tracking: Thanks to a customizable dashboard platform, it is easy to track each registration’s status at any point of time. Users know if registrations are falling behind schedule and how much budget is being spent.
- Collaboration: Functionalities such as document sharing and task alert system allow greater communication and collaboration among registration teams.
- Data Traceability: Data is stored via EUPHOR throughout the registration process facilitating future audits and business programs.
EUPHOR allows our clients to transform the challenge of compliance programs like REACH 2018 into an opportunity for improved productivity and business processes. It has helped Product Stewardship add value to the organization by providing the digital tool to create a robust compliance foundation for business growth and innovation.
www.euphoreach.com
info@euphoreach.com
+1 (732) 322 7220