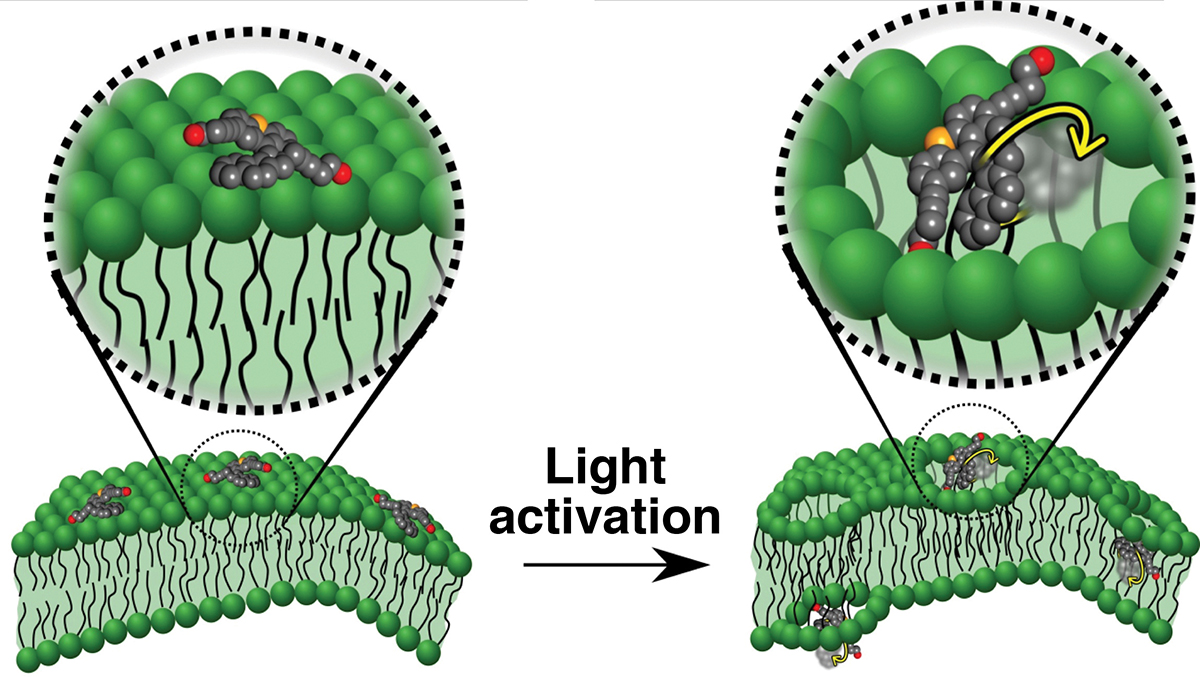
Motorised molecules driven by light have been used to drill holes in the membranes of individual cells and, according to the team behind the work, show promise for either bringing therapeutic agents into the cells or directly inducing the cells to die.
Researchers at Durham University, working with teams from Rice and North Carolina State universities in America, demonstrated in lab tests how rotors in single-molecule nanomachines can be activated by ultraviolet light to spin at 2 to 3 million rotations per second and open membranes in cells.
The researchers used motors based on work by Nobel laureate Bernard Feringa, who won the prize for chemistry in 2016.
The motor itself is a paddle-like chain of atoms that can be prompted to move in a single direction when supplied with energy. Properly mounted as part of the cell-targeting molecule, the motor can be made to spin when activated by a light source.
Leading the work was chemists James Tour of Rice, Robert Pal of Durham and Gufeng Wang of North Carolina State.
Their labs collaborated to create several motorised molecules that can home in on specific cells.
The Tour lab previously demonstrated molecular motors whose diffusion in a solution was enhanced, if not specifically directed, when activated by ultraviolet light. The rotors needed to spin between 2 and 3 megahertz – 2 to 3 million times per second – to show they could overcome obstacles presented by adjacent molecules and outpace natural Brownian motion.
James Tour said: “We thought it might be possible to attach these nanomachines to the cell membrane and then turn them on to see what happened.
“The motors, only about a nanometer wide, can be designed to target and then either tunnel through a cell’s lipid bilayer membrane to deliver drugs or other payloads or disrupt the 8-10 nanometer-wide membrane, thereby killing the cell. They can also be functionalized for solubility and for fluorescent tracking.
“These nanomachines are so small that we could park 50,000 of them across the diameter of a human hair, yet they have the targeting and actuating components combined in that diminutive package to make molecular machines a reality for treating disease.”
The researchers found it takes at least a minute for a motor to tunnel through a membrane.
Dr Pal, A Royal Society Research fellow, said: “We are moving towards realising our ambition to be able to use light-activated nanomachines to target cancer cells such as those in breast tumours and skin melanomas, including those that are resistant to existing chemotherapy.
“Once developed, this approach could provide a potential step change in non-invasive cancer treatment and greatly improve survival rates and patient welfare globally.”
The Pal lab at Durham tested motors on live cells, including human prostate cancer cells. Experiments showed that without an ultraviolet trigger, motors could locate specific cells of interest but stayed on the targeted cells’ surface and were unable to drill into the cells. When triggered, however, the motors rapidly drilled through the membranes.
James Tour said: “The researchers are already proceeding with experiments in microorganisms and small fish to explore the efficacy in-vivo. The hope is to move this swiftly to rodents to test the efficacy of nanomachines for a wide range of medicinal therapies.”
Rice graduate student Victor Garcia-López is the lead author of the study. Co-authors are graduate students Lizanne Nilewski and Amir Aliyan; research scientist Guillaume Duret; Anatoly Kolomeisky, a professor of chemistry and chemical and biomolecular engineering; and Jacob Robinson, an assistant professor of electrical and computer engineering, all of Rice; and North Carolina State alumnus Fang Chen. Robert Pal is a Royal Society University Research Fellow at Durham (U.K.). Gufeng Wang is an assistant professor of analytical chemistry at North Carolina State. James Tour is the T.T. and W.F. Chao Chair in Chemistry as well as a professor of computer science and of materials science and nanoengineering at Rice.
The National Science Foundation, North Carolina State University, the Royal Society and the Biophysical Sciences Institute at Durham University supported the research.